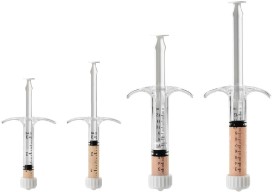
CuriGraft® Viable Allogenic Bone Matrix is the next generation allograft containing the three key elements ideal for bone formation, offering a variety of clinical applications, including the spine, upper extremity, foot and ankle and orthopedic oncology.
Key Features
-
-
- Osteoconductive three-dimensional scaffold with cortical and cancellous components
- Demineralized cortical bone scaffold. Demineralized cortical bone has been identified to have osteoinductive potential1
- Viable endogenous bone cells to support osteogenic healing processes
- DMSO-FREE cryoprotectant, requiring no rinsing or decanting
- Proprietary mixture of mineralized cancellous bone along with demineralized cortical fibers
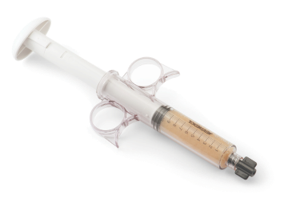
- Easy-to-use syringe, just thaw and use!
- Four-hour working window for implantation post thaw
- Storage: -65°C or colder
| Size |
Product Code |
| 1.0cc |
C034-0001-MCBMV |
| 2.5cc |
C034-0025- MCBMV |
| 5.0cc |
C034-0005- MCBMV |
| 10.0cc |
C034-0010- MCBMV |
Reference: 1) Gruskin, E. et.al., Demineralized bone matrix in bone repair: history and use. Advanced Drug Delivery Reviews, 2012. 64:1063-1077 Biologics
 Key Features
Key Features Key Features
Key Features














 System Features
System Features Key Features
Key Features Key Features
Key Features Key Features
Key Features Key Features
Key Features




 Key Features
Key Features Key Features
Key Features Key Features
Key Features